Measuring instruments and all-in-one solutions for storage facilities
When storing pharmaceuticals, a high degree of compliance awareness is required in order to work safely and in accordance with the standards. This is because the indoor climate in storage rooms has an effect on the efficacy and quality of pharmaceuticals and, thus, on people’s health.
Testo’s all-in-one solutions, data logger systems and data loggers ensure that the climate of your storage facility is kept constant. This enables you to respond to any deviations in good time and to reliably comply with the regulations covering GSP (Good Storage Practice), GDP (Good Distribution Practice) and 21 CFR Part 11.
Application example: GxP-compliant storage
Find out what’s important when it comes to storing pharmaceuticals: Download application example now.
IAQ monitoring: Stationary monitoring of environmental parameters
The all-in-one solution: The testo Saveris Pharma environmental monitoring system
Uninterrupted monitoring during storage – using one system.
- Integrated system comprising sensors, software and services
- Seamless recording and documentation of all audit-relevant IAQ parameters when storing pharmaceuticals
- Compliant with GxP and 21 CFR Part 11
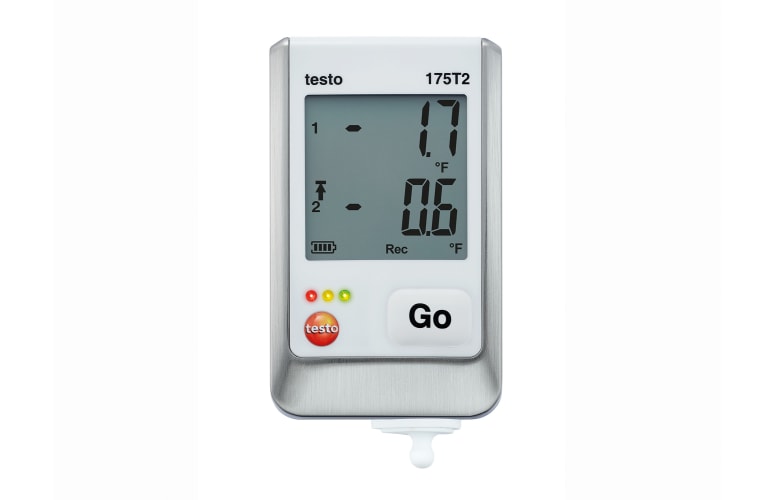
testo Saveris 2 radio data logger system
The testo Saveris 2 radio data logger system monitors and documents the ambient conditions in your storage facility.
- Measurement data access via cloud
- Individually positionable and extendable
- Alarm function
No effort, no gaps, no worries: Qualification and mapping of your storage areas
Critical temperature ranges in storage
Close to window and skylight
Distance to the ventilation outlet
Distance to the ventilation outlet
Directly at ventilation outlet
The storage of temperature-sensitive goods is a responsible task regulated by strict specifications.
Data loggers and services from Testo support you in fulfilling these conscientiously.
Goods and products that are sensitive to temperatures must be manufactured, stored and transported in qualified facilities, with qualified equipment and using validated processes.
Here, the three sub-processes of validation, qualification and mapping are of particular importance.
The purpose of validation is to ensure that a manufacturer's expected results can be guaranteed. A validation process takes place in three steps:
- Definition of the expected results and acceptance criteria.
- Verification and documentation that the process delivers the expected result.
- Determination of continuity, repeatability and accuracy of the validated process.
Qualification is the process of demonstrating that facilities and equipment are functioning properly.
For example, the qualification of a refrigerator or a high-bay warehouse ensures that all prescribed environmental conditions are met. Products that require refrigeration or are unstable pose particular challenges when it comes to storage.
A mapping (also called temperature or climate mapping) is part of this process step.
Temperature mapping identifies the zones of your storage, refrigeration and freezer areas that are within safe parameters for holding your products.
Mapping furthermore determines the performance limits of the air-conditioning systems. It also serves to identify critical control points in the refrigerator or storage area.
This enables you to optimally position the measuring points in your monitoring system and keep an eye on all the key parameters.
Expert report: Environmental monitoring during storage and transport of pharmaceuticals
In this technical report, our experts have prepared for you which legal and technical requirements are relevant in pharmaceutical logistics and which technical monitoring mechanisms you can use to reliably ensure seamless environmental monitoring.
Temperature and climate mapping with measurement technology from Testo: Your key benefits
- Highly precise from -200 to +1000 °C
- Comprehensive alarm management
- Models up to protection class IP68
- Measurement data storage, even with empty battery
- Uninterrupted recording and documentation of your measurement data
- Memory for up to 2 million readings
- Up to 8 years battery life
- Extensive probe range
- Brand quality from standard model to custom-made
Sensors & services: Mapping expertise from Testo
- Support with expertise, measurement technology and documentation
- For example for thermal sterilization, storage and transport
- Individual service packages for your requirement
Testo Industrial Services: Your service partner for transport and warehouse qualification
Storage and transport qualification ensures uniform temperature and environmental conditions. These are a prerequisite for maintaining the quality of your products during storage and the logistics process.
Testo Industrial Services supports you in monitoring and ensuring uniform environmental conditions in your warehouse or transport system. From individual temperature distribution measurements or metrological testing to project coordination and conceptual design of the entire qualification project.
Other areas of application
Find out more about the measuring solutions that can support you in your laboratory, cleanroom and production processes.
Full service for your logistics processes
Testo Industrial Services GmbH supports you in the form of expertise, measuring technology and documentation.
- Mappings for thermal sterilization, storage and transport.
- Service packages specifically adapted to your requirements
- For pragmatic implementation of the requirements placed on you
Gain a competitive edge when it comes to carrying out measuring tasks:
- Testo white paper IAQ monitoring
- Testo GxP Dictionary
- Plus lots more downloads
Which data logger is best for your application?
Download the comparison brochure now.
Do you require a personal consultation?